Pembrolizumab, a type of immunotherapy known as a PD-1 inhibitor
The news about pembrolizumab offering a survival benefit as an adjuvant treatment for kidney cancer is indeed a significant development in the field of oncology. Pembrolizumab, which is a type of immunotherapy known as a checkpoint inhibitor, works by blocking the PD-1 protein on the surface of immune cells, thereby enabling the immune system to better recognize and fight cancer cells.
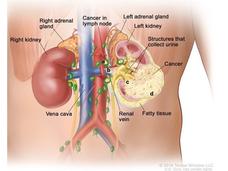
In the context of kidney cancer, particularly renal cell carcinoma (RCC), which is the most common type of kidney cancer in adults, the standard treatment has typically involved surgery to remove the tumor or the entire kidney, known as nephrectomy. However, there has been a lack of effective adjuvant therapies (treatments given after the primary treatment to increase the chances of a cure) to reduce the risk of recurrence in high-risk patients.
The clinical trial you are referring to would have been a randomized, placebo-controlled, double-blind study to provide the highest level of evidence for the efficacy of pembrolizumab in this setting. The key findings from such a study would likely include:
1. **Overall Survival**: The primary endpoint of many cancer trials is overall survival (OS), which refers to the length of time from either the date of diagnosis or the start of treatment that patients are still alive. If pembrolizumab shows a statistically significant improvement in OS, it means that patients receiving the drug are living longer than those who received the placebo.
2. **Disease-Free Survival**: Another important endpoint is disease-free survival (DFS), which measures the length of time after primary treatment that the patient survives without any signs or symptoms of that cancer. An improvement in DFS suggests that pembrolizumab helps to prevent the recurrence of kidney cancer after surgery.
3. **Safety Profile**: The safety and tolerability of pembrolizumab would also be a critical aspect of the study. While pembrolizumab is generally well-tolerated, it can cause immune-related adverse effects due to its mechanism of action. The trial would need to carefully monitor and report any side effects experienced by patients.
4. **Quality of Life**: Assessments of patients’ quality of life during and after treatment are increasingly recognized as an important aspect of cancer care. The trial might include measures of how the treatment impacts patients’ daily living and overall well-being.
If the trial’s results are robust and statistically significant, they could lead to a change in clinical practice, making pembrolizumab a new standard of care for patients with kidney cancer post-surgery. This would be particularly important for patients with a high risk of recurrence, where the need for effective adjuvant therapies is most critical.
Before pembrolizumab could become a standard adjuvant treatment, the results would need to be reviewed by regulatory bodies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). They would evaluate the data for efficacy, safety, and overall benefit-risk ratio before potentially approving the drug for this new indication.
For healthcare professionals, patients, and stakeholders in the field of oncology, such findings would be a cause for optimism, as they could translate into improved outcomes for individuals affected by kidney cancer.
https://www.cancer.gov/news-events/cancer-currents-blog/2024/kidney-cancer-pembrolizumab-increases-survival